New Cove. Some kind of krona virus that chinese scientists from woohan are warning about.
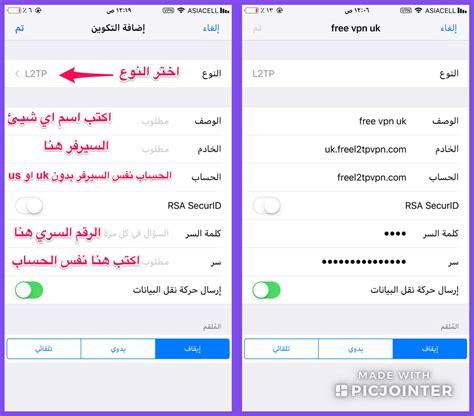
What's the NeoCove virus?
The "New Cove" virus is a virus detected in bats, and is the closest relative to the Middle East Respiratory Syndrome virus (MERS-CoV).
What's the Middle East respiratory syndrome?
The respiratory syndrome of the Middle East is a viral disease caused by a type of coronavirus called Mersekoff, which has been identified in many countries in the Middle East, Africa and South Asia. In total, 27 States have reported cases since 2012, resulting in 858 known deaths due to infection and related complications, according to the World Health Organization.
The origins of the virus are not fully known, but according to analysis of the various virus genomes, it is believed that it may have originated in bats and later moved to beauty at some point.
It is estimated that 35% of patients with the Middle East respiratory syndrome have died, i.e. the virus leads to the death of one in three infected persons.
Chinese scientists, mostly from Wuhan University, according to a report on today's Russia site.
The study was published on the website "Biorex" in pre-printing mode, which means it has not yet been accepted for publication in a court journal, and has not been reviewed by other scientists.
What did the Seekers find?
Researchers said they unpredictably found that "New Koff" and its initiator, the "PDF-2180-CoV", could efficiently use some of the types of angiogen-transferred enzyme 2 (ACE2) of the bats, and in a less favourable way, for the Angio-Palinan 2 human entry.
The researchers didn't say that, but they said that the main concern was whether the New Cove and BDF-2180-KOV viruses could jump the species barrier and humans.
What Are Scientists Afraid of the New Cove Virus?
Furthermore, the study shows that the current CAFID-19 vaccines are insufficient to protect humans from the risk of infection caused by these viruses.
What's the latest on CAFID-19?
Yesterday, Thursday, the European Union drug regulatory body authorized the use of the Visser anti-cofid tablets, in the first treatment of its kind to be taken through a licensed drug in Europe.
The European Agency for Pharmaceuticals said in a statement that it "recommended the use of Baxlovide for the treatment of Covered 19 in adults who are increasingly at risk of getting stronger symptoms of the disease."
The European Commission should now officially license the drug, in a procedure that normally takes hours or days.
Feiser's treatment of a mixture of a new compound, BF-07321332 (PF-07321332) and retonavir (ri Richardir) anti-HIV drug, is being taken as two tablets.
"The treatment of Paxlofide greatly reduces the chances of recovery or death for at least those already suffering from a disease, which exposes them to the risk of developing severe CFD-19 symptoms," the agency's experts said in a study.
Patients were given the pill within five days of symptoms, and in the following month only 0.8% of 1,039 people studied were hospitalized, compared to 6.3% of those who received a placebo.
There were no deaths in the group that received Paxlovid, compared to 8 deaths in the group that received the placebo.
Is there a need for a fourth dose of CAFID-19?
The French Council for the Guidance of Vaccines considered that there is currently no need to receive a fourth dose of the Kofid-19 vaccine, but it may revise its position if the scientific data are updated.
However, the only exception is for those "who are very vulnerable and who have been advised by the Council to take a second enhanced dose", according to a statement of the Council dated 19 January, reported by Agence France-Presse on Thursday.